Stevens Professors Discover a New Oxygen-Gold Structure
Interactions
between oxygen and gold are fundamentally important in multiple and diverse
areas of science and technology. Professors Fei Tian and Simon Podkolzin at the
Department of Chemical Engineering and Materials Science
discovered that in contrast with regular gold surfaces, oxygen interacts with
gold nanoparticles differently and forms a special structure. They discovered a
new oxygen-gold structure where two oxygen atoms are separated by a single gold
atom as Au-O-Au-O-Au. This discovery will advance the development of improved catalysts
with gold nanoparticles in chemical and petroleum-refining industries, improve
techniques for bio imaging of living cells, lead to better understanding of
spin-flip scattering processes of itinerant electrons in solid-state physics,
advance optical detection methods at the single-molecule level, advance the
development of better lithium batteries and improve understanding of
oxygen-gold interactions in other areas of science and technology.
|
|
|
Professors Simon Podkolzin (left) and Fei Tian at Stevens
Institute of Technology. |
Professors
Tian and Podkolzin reported their discovery in the publication entitled
“Observation and Identification of an Atomic Oxygen Structure on Catalytic Gold
Nanoparticles” DOI: 10.1002/anie.201706647 in Angewandte Chemie
International Edition in September 2017, journal
announcement on Twitter. The study was co-authored by graduate students Kai
Liu, Tao Chen, Shuyue He and Jason Robbins. Angewandte Chemie is the journal of
the German Chemical Society. The journal has been published since 1888, and it
is one of the oldest and most respected scientific publications. The work in
Prof. Podkolzin’s group was partially funded by the National Science Foundation
under Grant CBET-1264453. The work in Prof. Tian’s group was funded by the
American Chemical Society Petroleum Research Fund under Grant 55094-DNI5.
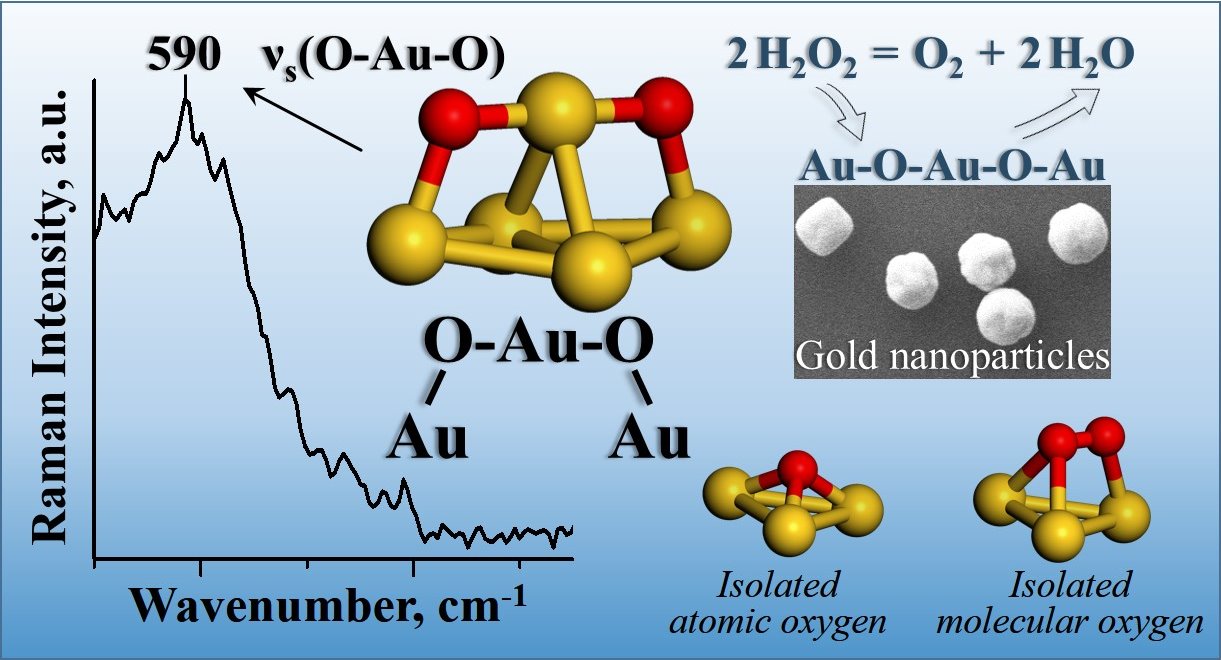
The
new oxygen-gold structure, Au-O-Au-O-Au, was observed and identified on gold
nanoparticles in catalytic decomposition of hydrogen peroxide to oxygen and
water. This structure with an oxygen dimer separated by a gold atom is
different from the known isolated atomic oxygen structures, which have only one
oxygen atom, and different from the known molecular oxygen structures, which
have two directly bonded oxygen atoms. The new structure was observed with in situ
surface-enhanced Raman spectroscopic measurements and identified with density
functional theory calculations. The experimental measurements were performed
using monodisperse 5, 50 and 400 nanometer gold particles supported on silica
with liquid-phase hydrogen and deuterium peroxides at multiple pH values. The
calculations show that on surfaces with coordinatively unsaturated gold atoms,
two oxygen atoms preferentially share a gold atom with a bond distance of
0.194-0.196 nanometers and additionally bind to two other surface gold atoms
with a larger bond distance of 0.203-0.213 nanometers, forming the Au-O-Au-O-Au
structure.
|
|
|
By studying catalytic decomposition of
hydrogen peroxide to oxygen and water over gold nanoparticles, Professors
Tian and Podkolzin discovered a new oxygen-gold structure where two oxygen
atoms are separated by a single gold atom. |
It
is challenging to characterize oxygen structures on gold surfaces because the
density of adsorption and reaction sites is typically extremely low. Flat gold
surfaces are usually chemically inert, and only a small fraction of gold
surface sites (coordinatively unsaturated sites, such as defects, steps and
kinks) serves as catalytically active sites. Professors Tian and Podkolzin were
able to address this challenge by utilizing ultra-high sensitive
surface-enhanced Raman spectroscopic measurements collected with a custom-built
system. An additional challenge is that properties of gold nanoparticles are
typically very dependent on their size. This second challenge was addressed by
evaluating monodisperse gold nanoparticles supported on silica. Monodisperse Au
nanoparticles are usually synthesized in colloidal solutions with stabilizing
organic ligands, which interfere with catalytic activity and spectroscopic
measurements. This third challenge was addressed by developing a simple thermal
treatment that removed stabilizing ligands, produced spectroscopically clean
surfaces, and yet allowed gold nanoparticles to remain mostly monodisperse on
the silica support.
Reaction
kinetic measurements showed that the newly discovered oxygen-gold structure is
not just a byproduct or a spectator species but an actual catalytic reaction
intermediate because it forms on the same gold atoms that serve as active sites
in catalytic decomposition of hydrogen peroxide. Therefore, this discovery will
advance the development of improved catalysts for selective oxidation reactions
that are urgently needed for sustainable and environmentally-friendly production
of chemicals and will be helpful in energy research, nanotechnology and in
numerous other fields of science and technology that rely on oxygen-gold
interactions.
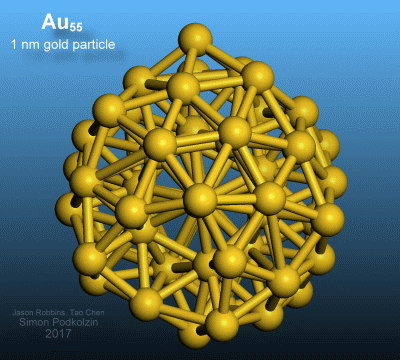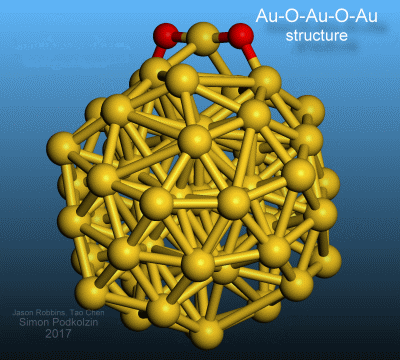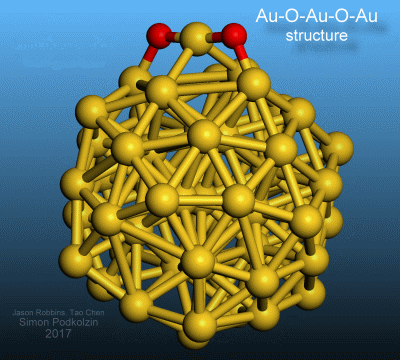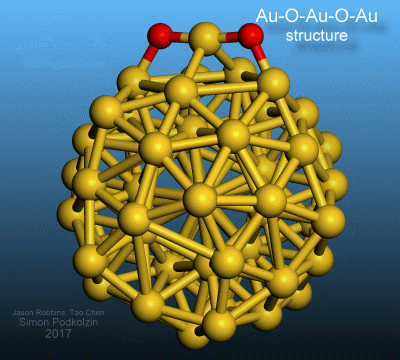
3-D visualization of the discovered oxygen-gold structure on a gold nanoparticle.